| References |
| Formal Name |
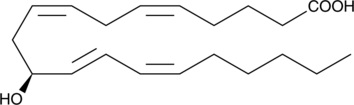
11S-hydroxy-5Z,8Z,12E,14E-eicosatetraenoic acid |
| CAS Number |
54886-50-9 |
| Molecular Formula |
C20H32O3 |
| Formula Weight |
320.5 |
| Formulation |
A solution in ethanol |
| Purity |
≥98% |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
CCCCC/C=CC=C/[C@@H](O)C/C=CC/C=CCCCC(=O)O
|
Background Reading
Myers, R.F., and Siege, M.I. Differential effects of anti-inflammatory drugs in lipoxygenase and cyclo-oxygenase activities of neutrophils from a reverse passive arthus reaction. Biochem Biophys Res Commun 112 586-594 (1983).
| Size |
Global Purchasing |
| 25 µg |
|
| 50 µg |
|
| 100 µg |
|
| 250 µg |
|
Description
The synthesis of 11-HETE by rat PMNL has been reported, but the stereochemistry of the 11-HETE produced is not defined.1 There are no definitive reports of a mammalian 11(S)-LO.
1
Myers, R.F., and Siege, M.I. Differential effects of anti-inflammatory drugs in lipoxygenase and cyclo-oxygenase activities of neutrophils from a reverse passive arthus reaction. Biochem Biophys Res Commun 112 586-594 (1983).
|