| References |
| Formal Name |
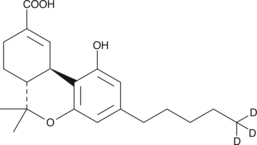
6aR,7,8,10aR-tetrahydro-1-hydroxy-6,6-dimethyl-3-pentyl-6H-dibenzo[b,d]pyran-9-carboxylic acid-5,5,5-d3 |
| CAS Number |
130381-15-6 |
| Molecular Formula |
C21H25D3O4 |
| Formula Weight |
347.5 |
| Formulation |
A solution in methanol |
| Purity |
≥99% deuterated product |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
CC1(C)[C@@H]2CCC(C(O)=O)=C[C@H]2C3=C(O)C=C(CCCCC([2H])([2H])[2H])C=C3O1
|
| Size |
Global Purchasing |
| 100 µg |
|
| 500 µg |
|
| 1 mg |
|
| 5 mg |
|
Description
(−)-11-nor-9-carboxy-Δ9-THC-d3 contains three deuterium atoms at the 5 position. It is intended for use as an internal standard for the quantification of (−)-11-nor-9-carboxy-Δ9-THC by GC- or LC-mass spectrometry. Δ9-Tetrahydrocannabinol (Δ9-THC), the active constituent of marijuana, is metabolized primarily by hydroxylation at the allylic C-11 position followed by oxidation to (−)-11-nor-9-carboxy-Δ9-THC. (−)-11-nor-9-carboxy-Δ9-THC is the major metabolite of Δ9-THC and is used as an internal standard in various analytical procedures to unequivocally confirm its presence in biological fluids.1
1
Siegel, C., Gordon, P.M., Uliss, D.B., et al. Synthesis of racemic and optically active Δ9-tetrahydrocannabinol (THC) metabolites. J Org Chem 56(24) 6865-6872 (1991).
|