服务热线
021-60498804
产品中心
/ Products Classification 点击展开+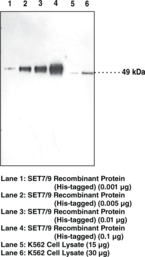
| Cat. Number | 069055463545088 |
||||||||||||||||||||
| Chemical Name | SET7/9 (FL) Polyclonal Antibody |
||||||||||||||||||||
| References |
Background ReadingKwon, T., Chang, J.H., Kwak, E., et al. Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9- Couture, J., Collazo, E., Hauk, G., et al. Structural basis for the methylation site specificity of SET7/9. Nat Struct Mol Biol 13(2) 140-146 (2006). Bhaumik, S.R., Smith, E., and Shilatifard, A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 14(11) 1008-1016 (2007). Kume, A., Miyazaki, T., Kitamura, Y., et al. High levels of saturated very long- Xiao, B., Jing, C., Wilson, J.R., et al. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature 421 652-656 (2003). Show all 5 Hide all but first 3
Description
Antigen:
human recombinant SET7/9 (amino acids 1-
1 Bhaumik, S.R., Smith, E., and Shilatifard, A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 14(11) 1008-1016 (2007). 2 Couture, J., Collazo, E., Hauk, G., et al. Structural basis for the methylation site specificity of SET7/9. Nat Struct Mol Biol 13(2) 140-146 (2006). 3 Xiao, B., Jing, C., Wilson, J.R., et al. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature 421 652-656 (2003).
4
Kwon, T., Chang, J.H., Kwak, E., et al. Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9- |
||||||||||||||||||||





