服务热线
021-60498804
产品中心
/ Products Classification 点击展开+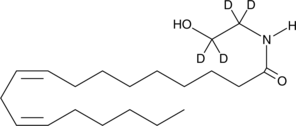
| Cat. Number | 653089805027580 |
||||||||||||||||||||||||||||
| Chemical Name | Linoleoyl Ethanolamide-d4 |
||||||||||||||||||||||||||||
| References |
Background ReadingBerdyshev, E.V., Schmid, P.C., Krebsbach, R.J., et al. Cannabinoid- Maccarrone, M., van der Stelt, M., Rossi, A., et al. Anandamide hydrolysis by human cells in culture and brain. J Biol Chem 273 32332-32339 (1998). Lin, S., Khanolkar, A.D., Fan, P., et al. Novel analogues of arachidonylethanolamide (anandamide): Affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J Med Chem 41 5353-5361 (1998). Watanabe, K., Matsunaga, T., Nakamura, S., et al. Pharmacological effects in mice of anandamide and its related fatty acid ethanolamides, and enhancement of cataleptogenic effect of anandamide by phenylmethylsulfonyl fluoride. Biol Pharm Bull 22(4) 366-370 (1999). Bisogno, T., Maurelli, S., Melck, D., et al. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem 272 3315-3323 (1997). Patrono, C., Rotella, C.M., Toccafondi, R.S., et al. Prostacyclin stimulates the adenylate cyclase system of human thyroid tissue. Prostaglandins 22(1) 105-115 (1981). Schmid, P.C., Kuwae, T., Krebsbach, R.J., et al. Anandanide and other N-
Description
Linoleoyl ethanolamide is an endocannabinoid detected in porcine brain and murine peritoneal macrophages which contains linoleate in place of the arachidonate moiety of arachidonoyl ethanolamide (AEA).1,2 It has weak affinity for the central cannabinoid (CB1) and peripheral cannabinoid (CB2) receptors, exhibiting Ki values of 10 µM and 25 µM, respectively.3 However, it is only approximately 4-
1 Patrono, C., Rotella, C.M., Toccafondi, R.S., et al. Prostacyclin stimulates the adenylate cyclase system of human thyroid tissue. Prostaglandins 22(1) 105-115 (1981).
2
Schmid, P.C., Kuwae, T., Krebsbach, R.J., et al. Anandanide and other N- 3 Lin, S., Khanolkar, A.D., Fan, P., et al. Novel analogues of arachidonylethanolamide (anandamide): Affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J Med Chem 41 5353-5361 (1998). 4 Watanabe, K., Matsunaga, T., Nakamura, S., et al. Pharmacological effects in mice of anandamide and its related fatty acid ethanolamides, and enhancement of cataleptogenic effect of anandamide by phenylmethylsulfonyl fluoride. Biol Pharm Bull 22(4) 366-370 (1999).
5
Berdyshev, E.V., Schmid, P.C., Krebsbach, R.J., et al. Cannabinoid- 6 Maccarrone, M., van der Stelt, M., Rossi, A., et al. Anandamide hydrolysis by human cells in culture and brain. J Biol Chem 273 32332-32339 (1998). 7 Bisogno, T., Maurelli, S., Melck, D., et al. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem 272 3315-3323 (1997). |
||||||||||||||||||||||||||||





