服务热线
021-60498804
产品中心
/ Products Classification 点击展开+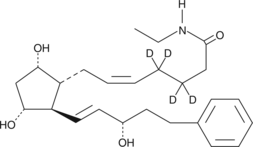
| Cat. Number | 652412803847856 |
||||||||||||||||||||||||||||||
| Chemical Name | 17-phenyl trinor Prostaglandin F2α ethyl amide-d4 |
||||||||||||||||||||||||||||||
| References |
Background ReadingLake, S., Gullberg, H., Wahlqvist, J., et al. Cloning of the rat and human prostaglandin F2α receptors and the expression of the rat prostaglandin F2α receptor. FEBS Lett 355 317-325 (1994). Balapure, A.K., Rexroad, C.E., Kawada, K., et al. Structural requirements for prostaglandin analog interaction with the ovine corpus luteum prostaglandin F2α receptor. Biochem Pharmacol 38 2375-2381 (1989). Maxey, K.M., Johnson, J., Camras, C.B., et al. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol 47(4) 34-40 (2002). Davies, S.S., Ju, W., Neufeld, A.H., et al. Hydrolysis of bimatoprost (lumigan) to its free acid by ocular tissue In Vitro. J Ocul Pharmacol Ther 19(1) 45-54 (2003). Woodward, D.F., Krauss, A.H., Chen, J., et al. The pharmacology of Bimatoprost (Lumigan™). Surv Ophthalmol 45 S337-S345 (2001). Sharif, N.A., Williams, G.W., and Kelly, C.R. Bimatoprost and its free acid are prostaglandin FP receptor agonists. Eur J Pharmacol 432 211-213 (2001). Show all 6 Hide all but first 3
Description
17-
1 Woodward, D.F., Krauss, A.H., Chen, J., et al. The pharmacology of Bimatoprost (Lumigan™). Surv Ophthalmol 45 S337-S345 (2001). 2 Maxey, K.M., Johnson, J., Camras, C.B., et al. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol 47(4) 34-40 (2002). 3 Balapure, A.K., Rexroad, C.E., Kawada, K., et al. Structural requirements for prostaglandin analog interaction with the ovine corpus luteum prostaglandin F2α receptor. Biochem Pharmacol 38 2375-2381 (1989). 4 Lake, S., Gullberg, H., Wahlqvist, J., et al. Cloning of the rat and human prostaglandin F2α receptors and the expression of the rat prostaglandin F2α receptor. FEBS Lett 355 317-325 (1994). |
||||||||||||||||||||||||||||||





