| References |
| Synonyms |
|
| Formal Name |
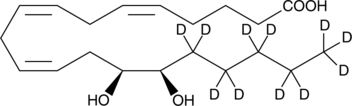
(±)14,15-dihydroxy-5Z,8Z,11Z-eicosatrienoic-16,16,17,17,18,18,19,19,20,20,20-d11 acid |
| Molecular Formula |
C20H23D11O4 |
| Formula Weight |
349.6 |
| Formulation |
A solution in ethanol |
| Purity |
≥99% deuterated product |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
OC(=O)CCC/C=CC/C=CC/C=CC[C@H](O)[C@H](O)C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])[2H]
|
Background Reading
Oliw, E.H., Guengerich, F.P., and Oates, J.A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem 257 3771-3781 (1982).
Catella, F., Lawson, J.A., Fitzgerald, D.J., et al. Endogenous biosynthesis of arachidonic acid epoxides in humans: Increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci USA 87 5893-5897 (1990).
| Size |
Global Purchasing |
| 25 µg |
|
| 50 µg |
|
| 100 µg |
|
| 250 µg |
|
Description
(±)14,15-DHET-d11 contains 11 deuterium atoms at the 16, 16, 17, 17, 18, 18, 19, 19, 20, 20, and 20 positions. It is intended for use as an internal standard for the quantification of (±)14,15-DHET by GC- or LC-mass spectrometry. Epoxide hydrolases convert the EETs into vicinal diols, with the concurrent loss of much of their biological activity.1 (±)14,(15)-DHET is the urinary metabolite of (±)14,(15)-EET which has been documented by GC-MS to be elevated in pregnancy-induced hypertension.2
1
Oliw, E.H., Guengerich, F.P., and Oates, J.A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem 257 3771-3781 (1982).
2
Catella, F., Lawson, J.A., Fitzgerald, D.J., et al. Endogenous biosynthesis of arachidonic acid epoxides in humans: Increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci USA 87 5893-5897 (1990).
|