| References |
| Synonyms |
|
| Formal Name |
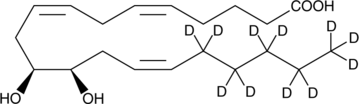
(±)11,12-dihydroxy-5Z,11Z,14Z-eicosatrienoic-16,16,17,17,18,18,19,19,20,20,20-d11 acid |
| Molecular Formula |
C20H23D11O4 |
| Formula Weight |
349.6 |
| Formulation |
A solution in ethanol |
| Purity |
≥99% deuterated product |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
| SMILES |
OC(=O)CCC/C=CC/C=CC[C@H](O)[C@H](O)C/C=CC([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])[2H]
|
Background Reading
Oliw, E.H., Guengerich, F.P., and Oates, J.A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem 257 3771-3781 (1982).
Fang, X., Kaduce, T.L., Weintraub, N.L., et al. Functional implications of a newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circ Res 79 784-793 (1996).
| Size |
Global Purchasing |
| 25 µg |
|
| 50 µg |
|
| 100 µg |
|
| 250 µg |
|
Description
(±)11,12-DHET-d11 contains 11 deuterium atoms at the 16, 16, 17, 17, 18, 18, 19, 19, 20, 20, and 20 positions. It is intended for use as an internal standard for the quantification of (±)11,12-DHET by GC- or LC-mass spectrometry. Epoxide hydrolases convert the EETs into vicinal diols, with the concurrent loss of much of their biological activity.1 11,12-DHET relaxes U-46619-contracted artery rings with approximately 70% of the magnitude of 11,12-EET.2
1
Oliw, E.H., Guengerich, F.P., and Oates, J.A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem 257 3771-3781 (1982).
2
Fang, X., Kaduce, T.L., Weintraub, N.L., et al. Functional implications of a newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circ Res 79 784-793 (1996).
|